Integrating Special Populations
Integrating special populations into clinical research.
Geriatrics
Director and Contact person: Holly Holmes, MD - [email protected] - 713-500-6628
The CCTS has access to diverse populations of older adults across its catchment areas with significant burden of chronic illness. Recent advancements include a study led by Dr. Holmes evaluating whether a pharmacist-led deprescribing intervention can decrease adverse drug reactions, healthcare utilization, and cost. The CCTS has also funded pilot studies for new investigators, such as one conducted by Dr. Jessica Lee in collaboration with UTMB and UT San Antonio, examining the effects of intranasal oxytocin on sarcopenic obesity. Other studies include the AstraZeneca COVID-19 vaccine trial with co-investigators Dr. Lee and Dr. Carmel Dyer. Dr. Lee is a site PI and supervisor for a trial of statins for primary prevention of dementia and disability (PREVENTABLE). Dr. Lee has also formed unique partnerships with community organizations. She is conducting an exercise clinical trial for homebound older adults in collaboration with the community organization Meals on Wheels. Another longitudinal study in Brownsville, with the CCHS, has recruited more than 400 primarily elderly participants which has helped uncover new associations between chronic diseases in older people, for example diabetes and osteoporosis. Dr. Rianon works in close collaboration with the UT SPH in Brownsville in these studies.

Dr. Jessica Lee, a geriatrician with UTHealth, is conducting a Phase III clinical trial to assess if a potential vaccine is effective at preventing symptomatic COVID-19. (Contributed/UTHealth)
Helpful links
UTHealth Joan and Stanford Alexander Division of Geriatric and Palliative Medicine
National Clinical Trials Database
Pediatrics
Director: Jon Tyson, MD, MPH - [email protected] - 713-500-5651
Dr. Jon Tyson leads a team of CCTS investigators in conducting innovative and rigorous research for patients from the fetal stage up to age 18. Among their contributions, Claudia Pedroza, PhD, Charles Green, PhD, and Dr. Tyson have been leaders in pioneering the use of Bayesian design and analysis of clinical trials, particularly perinatal and pediatric studies, to address the limitations of traditional frequentist statistics and to quantify the probability and magnitude of benefit/harm from medical or surgical interventions. This work includes assisting in perinatal studies led by Sean Blackwell, MD, Suneet Chauhan, MD, and other maternal-fetal medicine specialists. Another CCTS investigator, Joyce Samuel, MD, MS, has led in promoting the use of n-of-1 trials as a method to identify the best treatment for individual patients, including children with hypertension whose response to different medications is quite variable and unpredictable. Ricardo Mosquera, MD, MS, and Elenir Avritscher, MD, PhD, MBA, have developed a nationally renowned program for medically complex children and demonstrated its effectiveness and cost effectiveness in progressively reducing their rates of serious illnesses and health system costs.

Dr. Mosquera works with a patient through telemedicine.
Drs. Tyson, Pedroza, and Green have been leaders in randomized trials and cohort studies of the NICHD Neonatal Research Network that are needed to resolve major controversies in the care of high-risk newborns and are often published in The New England Journal of Medicine and JAMA. These include the study of Dr. Tyson and Green that developed an internet tool to assess the prognosis of marginally viable newborns and assist in counseling the parents of these infants. Matthew Rysavy, MD, PhD, and Dr. Tyson recently updated this tool, which is accessed more than 30,000 times per year by neonatologists and parents in developed countries worldwide. Drs. Tyson and Rysavy are now leading several major Network studies, including a trial of cycled phototherapy to increase the likelihood of survival without impairment among extremely premature infants.
To participate in similar perinatal and pediatric studies or to apply research methods of the kind noted above in studies of patients of any age, please contact Dr. Tyson.
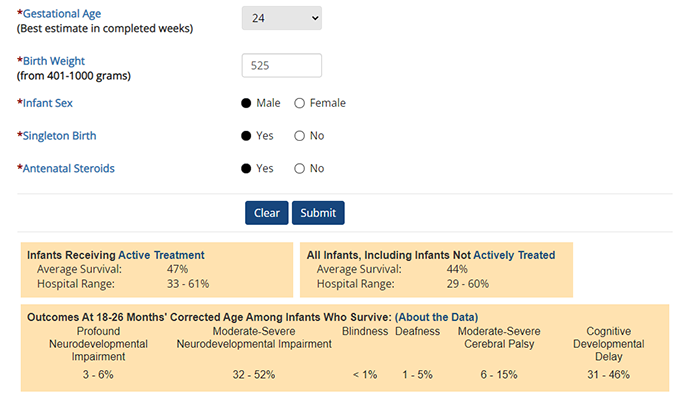
The online neonatal estimator allows provides detailed information on an infant's prognosis.
Hispanics with Cancer
Directors and Contact persons: Susan P. Fisher-Hoch, MD - [email protected]; Joseph B. McCormick, MD - [email protected] - 956-755-0605
A unique feature of the CCTS is the large representation of a low-income Hispanic population. Houston is 37.4% Hispanic, and the CCTS Brownsville CRU region is over 90% Hispanic. The CCTS utilizes this strong relationship with the Hispanic community to support the Cameron County Hispanic Cohort (CCHC, N>5000). This randomized cohort of mostly Mexican-Americans, mostly underserved in terms of both education and health, is actively recruited for clinical research at the Brownsville Clinical Research Unit (CRU).
The research made possible by the cooperation of CCHC participants has resulted in broad based and unique medical discoveries. Among the most important is the incidence of liver disease and cancer mostly associated with diabetes and obesity. Collaborative research with Dr. Laura Beretta of M. D. Anderson Cancer Center has revealed a high prevalence of liver disease, a precursor to liver cancer driven by obesity, and poorly controlled diabetes and a lack of access to health care. Underlying genetic predisposition is also being explored.
Much of the CCHC recruitment is achieved through direct, personal approaches to households to acquire and retain the trust of this infrequently studied community. The Brownsville CRU has state-of-the-art resources and imaging equipment, especially for liver disease, and an inviting atmosphere for these CHCC participants. The CRU in Brownsville is now open at full capacity following COVID-19 guidelines.
This research has provided solid and detailed data on a range of underlying chronic conditions in the population, including obesity, diabetes, hypertension, cardiovascular disease, mental health, and cognitive function, among others. Such published data support efforts at health education and disease management programs such as Salud y Vida, which helps patients manage uncontrollable diabetes and hypertension. The high prevalence of chronic disease and the low access to primary health care has also spurred the development of a program of telehealth to provide primary care to those without a primary care provider.

CRU and lab team SPH Brownsville campus.
Lesbian, Gay, Bisexual, Transgender, and Queer+ (LGBTQ+) Community
Director and Contact person: Vanessa Schick, PhD - [email protected] - 713-500-9398
The LGBTQ+ community is diverse, and individuals are often missed when the community is not appropriately engaged. The CCTS has a history of supporting community-engaged research, including awarding mini-grants to increase community capacity in LGBTQ+-serving organizations. Please read more about the Community Health Initiated Research Partnerships (CHIRP) Fellowship program here. [link to CE]
Inappropriate engagement of the community is a major barrier to participation in research. Specifically, LGBTQ+ members are often lost to studies when the questions asked do not accurately capture their sexual orientation or gender identity. The CCTS is a resource for individuals with questions about how to properly ask these questions. The team, including the UTHealth LGBTQ+ student group, developed a 1-pager for researchers and clinicians on how to address these issues. A draft version is available here: LGBTQ+ Research & Clinical Practice Recommendations.
Individuals within the LGBTQ+ community are often hesitant to engage in research for a variety of reasons (e.g., historical distrust). For this reason, the community may be better engaged by offering needed services outside of research. Dr. Schick and her team worked with community partners to scale a tailored, interactive resource guide (Personalized Assessment List (PAL)) to provide individuals with a list of local, available resources related to COVID-19, healthcare, and social determinants of health (e.g., housing, food insecurity, housing) designed to meet their individualized needs and characteristics:
HOUSTON LGBTQ+ PAL [Personalized Assessment List] Are you a member of the LQBTQ+ community interested in being involved in clinical trials or research? Many opportunities will provide financial compensation for your time and participation. The PAL has an option for you to sign-up for clinical trials when they become available.
Are you a researcher or practitioner who has an opportunity to share with the LGBTQ+ community? Send an email to [email protected] to review your opportunity and/or to send it to the individuals who signed up for notifications through PAL.