In 2006, three Texas Medical Center institutions were among the first in the nation to receive a Clinical and Translational Science Award (CTSA) from the National Institutes of Health to accelerate the translation of laboratory discoveries into patient treatments. The grant was used to establish the Center for Clinical and Translational Sciences (CCTS). Headquartered at UTHealth Houston, the CCTS includes the following partner organizations and campuses:
- UTHealth Houston
- The University of Texas MD Anderson Cancer Center
- UT Tyler Health Science Center
- The University of Texas Rio Grande Valley
- Rice University
- Texas Tech Health El Paso
- Memorial Hermann Health System
- Harris Health Lyndon B. Johnson Hospital
CCTS resources are available to all faculty, staff, and trainees at each of the above institutions.
What is Translational Research?
Translational research refers to the process of moving findings from the lab to the clinic to the community, and moving community concerns and opinions to the clinic and the lab. Translation may seem like an automatic part of research and medical practice, but in reality it is a major stumbling block in science, medicine, and public health. This is partly due to the compartmentalized history of research training and of the public’s role in biomedical research. Until the advent of the Clinical and Translational Science Award program (CTSA), basic scientists were not generally trained to think of the clinical application of their work, clinicians were often not taught to formulate research studies based on clinical observations, and public health scientists and community members may not have gotten a strong background in basic or clinical research (but had the knowledge of the community the other two groups may have lacked). These four groups have long collaborated, but as our knowledge has grown and research becomes more complicated, it has become apparent that new ways of approaching basic health problems are needed for seamless translation. Funded by the National Institute of Health (NIH), the CTSA program is a pivotal element that will accelerate translation.
The Process of Translational Research
Clinical and translational research can be divided into five stages. Importantly, translational is bidirectional across the stages.
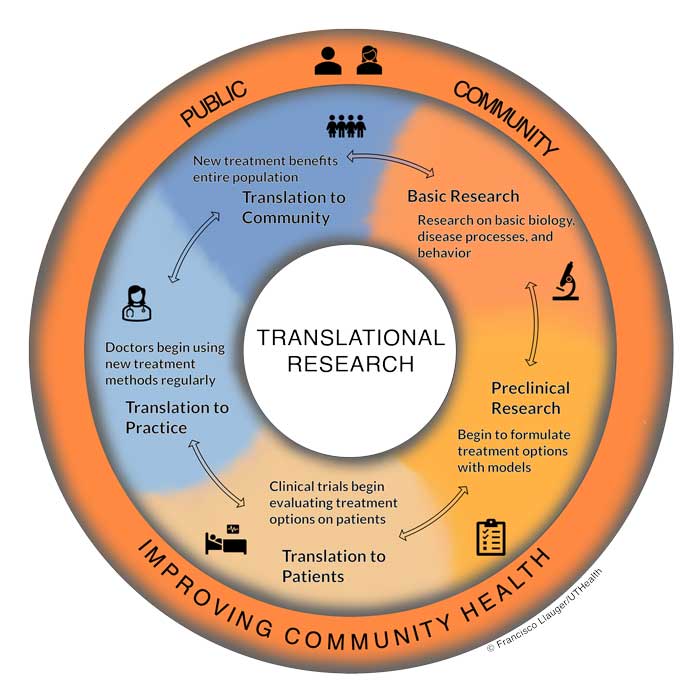
Basic research involves investigations in the basic biology, disease processes, or behavior. The discovery of new cancer-causing mutations or genes influencing addiction and substance abuse are potential examples of discoveries from basic research.
Preclinical research takes the findings of basic research and places them in the context of diagnosis and treatment through the use of animal models or cultured human cells. These findings help scientists begin to understand how diagnosis and treatments may be developed and work out potential issues without involving patients directly.
Translation to patients uses clinical trials to allow diagnosis and treatment options to be implemented in real patients. Clinical trials are carefully planned to be safe while still generating important results whether treatments work or not. The results of clinical trials are then used to seek regulatory approval for wide implementation of a treatment. For example, all COVID-19 vaccines underwent vigorous clinical trial testing before being offered to the general public.
Translation to practice occurs when clinicians and medical practices begin regularly using the new treatment or diagnosis option on their patients. During this stage, doctors can make note of any issues or deficiencies in the treatment that may not have been detected yet, or begin to identify new questions, to back translate” in the translational research process for further research.
Translation to community occurs when all community members, researchers, doctors, and patients are aware of and benefiting from the new treatment options. This is the ultimate goal of translational research in improving community health. .
It would be ideal for these stages to be concrete with well-defined interactions. However, in practice translational research is more of a spectrum whereby researchers, clinicians, and the public collaborate in multiple contexts. Every stage allows for communicating back to the previous step or across the spectrum to include a completely different stage should more research be needed-the public may suggest research studies, researchers may need access to patients for basic research, doctors may involve community members before implementing new treatment options, and many more interactions may occur that are more nuanced than any diagram can indicate. However, the central idea and goal remain the same: to facilitate communication between researchers, doctors, and the community to identify health concerns, research new treatments, and implement care to improve community health.
Who can use the CCTS?
The Public: See our Public Page for more information.
El público: Visita nuestra Página Pública para más información.
Faculty, trainees, and staff of our participating institutions (The University of Texas Health Science Center at Houston (UTHealth), The University of Texas M D Anderson Cancer Center, The University of Texas Health Science Center at Tyler, The University of Texas Rio Grande Valley, and Rice University) can use the services of the CCTS Components.
We assist other investigators as we are able. Email your request to CCTS@uth.tmc.edu for information.
How Can We Help You?
Many of our services are free, and others are offered at cost. All initial consultations are completely free. To use our services if you are a member of our participating institutions:
First, read the list of our components below.
Click on the ones that interest you for a complete description.
Then, decide whether you need or want a consultation.
If you're an experienced researcher and don't need to discuss study design and CCTS services with our staff, then you can contact the individual CCTS components directly. If you have questions, you're a new investigator or new to the Texas Medical Center, or you just want to explore how to expedite your research project, call our main line (713-500-7900) or email CCTS@uth.tmc.edu.