eRA Commons is an online interface where grant applicants, grantees, and federal staff at NIH and grantor agencies, such as AHRQ and CDC, can access and share administrative information relating to research grants.
The functions available to a user in eRA Commons are based on the 'role' associated with their eRA Commons account.
To gain access to eRA Commons:
- Fill out our Account Access Form and send it to [email protected]
*Please note: Further explanation of roles below
-
Roles
Administrative Roles for Research Support
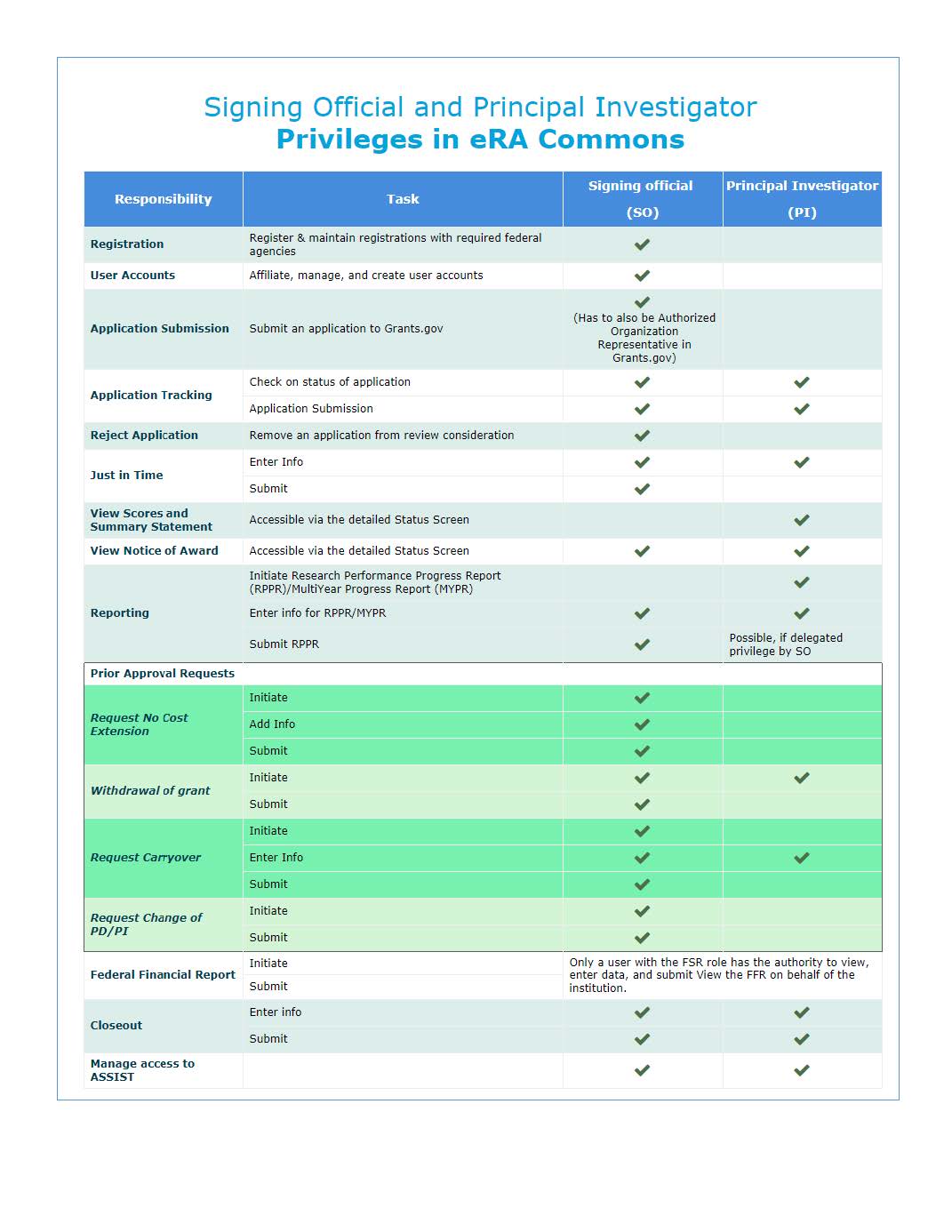
Signing Official (SO)
A Signing Official (SO) has institutional authority to legally bind the institution in grant-administration matters by providing signature approval on grant application submissions. The SO monitors grant related activities within the extramural organization and may have a number of titles.
A Signing Official (SO) role has the following privileges:
- Register the applicant institution in the eRA Commons
- Edit and maintain the Institutional Profile (IPF)
- Create/delete/update all Commons accounts (except IAR and TRAINEE accounts)
- Create affiliation of an existing Principal Investigator (PI) and/or IAR Commons account to their institution
- Update his/her own Personal Profile
- Submit and reject applications
- View application image and status for all submitted applications within the organization
- View award information (not the Summary Statement or the Score)
- Edit and submit JIT (Just-in-Time) requests
- Edit, route, and submit Research Performance Progress Report (RPPR), if they are the current reviewer
- Can delegate submit authority to PI* for the PI's RPPRs
- Request for No Cost Extension (NCE)
- Submit closeout documents (must have FSR role for the Final Financial Report [FFR])
- In xTrain, view:
- trainee roster
- list of grants
- grant summary
- routing history
- PDF Appointments/Amendments/Termination Notices
- In xTRACT:
- View tables
- Create xTRACT persons
*Please note: A Principal Investigator (PI) who is delegated to submit RPPRs becomes the institutional authority to legally bind the institution with regards to the submission of that RPPR.
Additionally, Signing Officials can:
- Only be affiliated with one organization
- Be combined with other administrative roles but cannot be combined with scientific roles
- Modify existing accounts (updating email address associated with account and add/remove roles) within their organization
Administrative Official (AO)
An Administrative Official (AO) within an extramural organization may be located within the Central Research Administration Office and/or an academic department. The AO reviews grant applications for accuracy before the SO submits final applications to the NIH. Depending on an institution's workflow process, it is possible for the SO and AO to be the same person. In this case, only SO authority is necessary (as SO authority supersedes AO authority). SO and AO authorities should not be combined.
An Administrative Official (AO) role has the following privileges:
- View the Institutional Profile, but not modify it
- Update his/her own Personal Profile
- Create all accounts except SO, BO, FCOI accounts, and IAR accounts
- Create affiliation of an existing PI or IAR Commons account to their institution
- View status and award information for all institution grants (not the Summary Statement or the Score)
- Edit and route Research Performance Progress Report (RPPR), if they are the current reviewer
- In xTRACT:
- View tables
- Create xTRACT persons
*Please note:: An Administrative Official (AO) is not authorized to submit applications or reports to NIH
Additionally, Administrative Officials can:
- Only be affiliated with one organization
- Be combined with other administrative roles but cannot be combined with scientific roles
- Modify existing accounts (updating email address associated with account and add/remove roles) within their organization
Account Administrator (AA)
Designated by the SO, the Accounts Administrator (AA) facilitates the administration of eRA Commons accounts. The AA typically is located in the Central Research Administration Office at the grantee organization.
An Accounts Administrator (AA) role has the following privileges:
- View the Institutional Profile, but not modify it
- Update his/her own Personal Profile
- Create all Commons accounts except SO, FCOI accounts, and IAR accounts
- Create affiliation of an existing PI Commons account
- In xTRACT:
- View tables
- Create xTRACT persons
Additionally, Account Administrators can:
- Only be affiliated with one organization
- Be combined with other administrative roles but cannot be combined with scientific roles
- Modify existing accounts (updating email address associated with account and add/remove roles) within their organization
Business Official (BO)
The Business Official (BO) role is used with xTrain; which is a service to electronically manage appointments for awarded Training Grants.
A Business Official (BO) role has the following privileges:
- View the Institutional Profile, but not modify it
- Update his/her own Personal Profile
- Create all accounts except SO, BO, FCOI accounts, and IAR accounts
- View the following Training-Grant-related items:
- Trainee Roster o List of Grants o Grant Summary o Routing History
- PDF-formatted Appointments/Amendments/Terminations
- Initiate, update, route, and submit Termination Notices (only for user authorized to submit TNs)
Additionally, Business Officials can:
- Only be affiliated with one organization
- Be combined with other administrative roles but cannot be combined with scientific roles
- Modify existing accounts (updating email address associated with account and add/remove roles) within their organization
Scientific Roles for Research
Principal Investigator (PI)
A Principal Investigator (PI) directs a research project or program supported by the NIH. The role of the PI within the eRA Commons portal is to complete the grant administration process or to delegate this responsibility to another individual. A PI may only access information pertaining to the grant(s) on which he/she is the designated PI.
NIH has adopted a Multiple-PI model — as directed by the Office of Science and Technology Policy — permitting more than one PI to be associated with an NIH-funded grant, contract, or cooperative agreement. Additional named PIs assist with the responsibilities currently accorded to a single PI. The multiple-PI model is intended to supplement — not replace — the traditional single-PI model.
A Principal Investigator (PI) role has the following privileges:
- View the Institutional Profile, but not modify it
- Update his/her own Personal Profile
- Delegate the authority for others at their institution with an eRA Commons account to edit their Personal Profile (PPF delegation)
- View status of all grant applications for which they are the designated Program Director/Principal Investigator (PD/PI), including any errors or warnings that may have been triggered
- View assembled image of submitted grant applications before they move on for further processing
- View Study Section/Meeting Roster of the Review Group that will be reviewing an application
- View Review outcome information and Summary Statements
- View Notice of Award (NOA) for all grants for which they are the designated PD/PI
- Delegate the ability for another user at their institution with an eRA Commons account to edit their Research Performance Progress Report (Progress Report delegation)
- Delegate the ability for another user at their institution with an eRA Commons account and the ASST role to view the Status information of an application, except outcome and Summary Statements (Status delegation)
- Delegate the ability for another user at their institution with an eRA Commons account and the ASST role to edit xTrain reports (xTrain delegation)
- Initiate an RPPR reports
- Complete the following Training-Grant-related items using xTrain:
- Initiate, update, route, and submit Appointments, Re-Appointments, and Amendments in xTrain
- Initiate, update, and route Termination Notices in xTrain o View Trainee Roster
- View List of Grants o View Grant Summary o View Routing History
- View PDF-formatted Appointments/Amendments/Terminations
*Please note:: A Principal Investigator (PI) should only ever have one eRA Commons account throughout the career of the investigator. Once the account is created, it can affiliated and unaffiliated with any number of institutions as needed.
Additionally, Principal Investigators can:
- Have multiple affiliations
- Can be combined with other scientific roles, but cannot be combined with administrative roles
*Please note:: For small institutions where a user may have both scientific and administrative responsibilities, they will need separate accounts for each type of activity.
Trainee
The eRA Commons xTrain Trainee role is used to electronically manage appointments for awarded Training Grants.
A Trainee role has the following privileges:
- View the Institutional Profile, but not modify it
- Update his/her own Personal Profile
- View his/her own PDF-formatted Appointments/Amendments/Terminations and their routing history
- Update and route his/her own Appointments/Amendments/Terminations
Sponsor
A sponsor supervises the research training experience of individual fellows supported by fellowship awards in the xTrain module.
A Sponsor role has the following privileges:
- View the Institutional Profile, but not modify it
- Update his/her own Personal Profile
- View the following Training Grant related items:
- Trainee Roster
- List of Grants
- Review Termination Notices and route to BO before submission to Agency
- Initiate Termination Notices on behalf of fellows who have left the institution
*Please note:: The Sponsor delegation can be assigned by the SO or AA to a user who as the ASST role. The delegation permits the user to access the xTrain module to review trainee rosters, view the list of grants, and review termination notices.
Other Important Roles
Assistant (ASST)
The Assistant (ASST) role is used in conjunction with specific delegations so the user can assist the PI with data entry and the completion of reports. The ASST user cannot be affiliated with more than one organization and it should be the same organization as the PI that is giving the delegation.
The ASST role has the following privileges:
- View the Institutional Profile, but not modify it
- Update his/her own Personal Profile
- Update the Personal Profile of a PI, if assigned by the PI the PPF delegation
- If assigned the Sponsor delegation by the SO or AA:
- Review trainee rosters
- View the list of training grants for the PI
- Initiate, review, and edit Appointments/Amendments/Termination notices
- If assigned the xTrain delegation by a PI or Sponsor:
- Review trainee rosters
- View the list of training grants for the PI
- Initiate, review, and edit Appointments/Amendments/Termination notices
- View PDFs of notices
- Initiate, view, and edit xTRACT tables
- Create xTRACT persons
- If assigned the Status delegation by a PI:
- View application image and status for all applications submitted by the delegating PI
- View award information
- Edit and save JIT (Just-in-Time)
- If assigned the Progress Report delegation by a PI:
- Initiate, edit, and route RPPRs
*Please note:: ASST users with the xTrain delegation cannot modify the Stipend field in a termination notice.
Internet Assisted Reviewer (IAR)
Specially selected by Scientific Review Officers (SRO) of the NIH, an Internet Assisted Reviewer (IAR) can critique and score submitted grant applications. Many PIs are selected for this role and IAR authority is automatically added to their account once an SRO enables them for a meeting. All other reviewers who have never served as PIs have IAR authority solely.
The ASST role has the following privileges:
- The IAR role does not have an affiliation
- View the Institutional Profile, but not modify it, if affiliated with an institution
- Update his/her own Personal Profile
- Access to IAR tab for meeting information
- Upload and submit critiques and preliminary scores for application to be reviewed at a meeting in which they are enabled.
*Please note: The Scientific Review Officer (SRO) is only person responsible for creating IAR accounts. The reviewer will need to be enabled or invited to a meeting in order to obtain the IAR role
ASSIST Access Maintainer (ASSIST_ACCESS_MAINTAINER_ROLE)
The newest role for eRA Commons is the ASSIST Access Maintainer role
(ASSIST_ACCESS_MAINTAINER_ ROLE). A user with this role has the authority to manage access to applications in ASSIST on behalf of the organization. Only the Signing Official (SO) has the authority to provide this role.
The ASSIST Access Maintainer role has the following privileges:
- View the Institutional Profile, but not modify it
- Update his/her own Personal Profile
- Manage access to all applications at the organization level, rather than application by application level
*Please note:: Access Management within ASSIST for individual applications has not changed.
Administrative Roles for Reporting
Financial Conflict of Interest (FCOI)
The Financial Conflict of Interest role should be assigned to the Commons user(s) in the institution who will manage the FCOI reporting process.
The Financial Conflict of Interest role has the following privileges:
- View the Institutional Profile, but not modify it
- Update his/her own Personal Profile
- Initiate, edit, submit, revise, view, and delete records and documents concerning FCOI
Financial Conflict of Interest Assistant (FCOI_ASST)
The FCOI_ASST role is assigned to those users in the institution who will assist in working on the FCOI reporting process.
The Financial Conflict of Interest Assistant role has the following privileges:
- View the Institutional Profile, but not modify it
- Update his/her own Personal Profile
- Initiate, edit, view, and delete records and documents concerning FCOI
Financial Conflict of Interest View (FCOI_VIEW)
The FCOI_VIEW role is assigned to those users who need authority to search for and view FCOI information entered by the institution in the FCOI module, but who will not perform any data entry or make changes to the information.
The Financial Conflict of Interest View role has the following privileges:
- View the Institutional Profile, but not modify it
- Update his/her own Personal Profile
- Search and view records and documents concerning FCOI
- Users have read-only access to FCOI report data.
*Please note: Only the SO can assign the FCOI roles.
Financial Status Reporter (FSR)
The Financial Status Reporter (FSR) is responsible for the reporting a statement of expenditures for a grant. Depending on the institution workflow process, it is possible for the SO and FSR to be the same person (these two authorities may be combined). If the FSR is a different individual, a separate FSR account must be created.
The Financial Status Reporter role has the following privileges:
- View the Institutional Profile, but not modify it
- Update his/her own Personal Profile
- Submit Financial Status Reports on behalf of the institution.
*Please note: An account with only the FSR role assigned can only perform FSR tasks. An account can include multiple administrative roles, including that of FSR.
Public Access Compliance Report (PACR)
The Public Access Compliance Report (PACR) role, although assigned through eRA Commons, provides access to Public Access compliance reports for their institution generated outside of eRA Commons.
The Public Access Compliance Report role has the following privileges:
- View the Institutional Profile, but not modify it
- Update his/her own Personal Profile
For more information see Public Access Compliance Monitor: A New Resource for Institutions to Track Public Access Compliance (NOT-OD-13-020).
Scientific Roles for Reporting
The following roles are for reporting purposes only. As part of the NIH effort to improve data on the Biomedical Workforce (BMW) initiative, users who participate in NIH-supported projects are required to have an eRA Commons account for reporting purposes.
The following roles have these privileges:
- Update his/her own Personal Profile
*Please note: It is important that for these accounts, the education section of the personal profile is up-to-date and accurate.
Undergraduate Student (UNDERGRADUATE)
Role assigned to an individual who is in an undergraduate program and is participating in an NIH-funded project for at least one person month.
Graduate Student (GRADUATE_STUDENT)
Role assigned to an individual who is in a graduate program and is participating in an NIH-funded project for at least one person month.
Post-Doctoral (POSTDOC)
The Post-Doctoral (PostDoc) role is part of the Manage Accounts dropdown list of roles that can have affiliations with multiple institutions. The Commons Electronic Trainee Activities Interface (X-Train) provides the means for NIH supported Post-Doctoral students and trainees to provide NIH with the information needed to administer their Ruth L. Kirschstein National Research Service Awards (Kirschstein-NRSA) training grants. Required information includes:
- Address
- Educational background
- NIH monetary support
The Training Activities (TA) module is used at the NIH to support business operations for processing training appointments, terminations, and payback obligations of NRSA trainees and terminations and payback obligations of Kirschstein-NRSA individual fellows. A signed hard copy of the Payback Service Agreement is required for first- time postdoctoral trainees appointed to a Kirschstein-NRSA research-training grant.
NIH awards these training grants every year to help individuals train for research careers in the behavioral, biomedical and clinical sciences. Under current legislation, postdoctoral recipients of Kirschstein-NRSA support must pay back the first 12 months of support either by time -in- service or through monetary remuneration. Additional information on the Kirschstein-NRSA can be found at http://grants.nih.gov/training/nrsa.htm
Scientist (SCIENTIST)
Use the Scientist role to identify an individual who is participating in an NIH-funded project for at least one person month.
Project Personnel (PROJECT_PERSONNEL)
Use the Commons Project Personnel role to identify an individual who is participating in an NIH-funded project for at least one person month.
- Signing Official & PI Privileges